Matthew Allen
You run the largest research lab at Harvard, the Church Lab, which carries out an extraordinary range of projects in synthetic biology. Your work has a lot in common with architecture; both are about altering human experience. If human experience plays out through the relationship between the body and the environment, perhaps synthetic biology and architecture influence our experience from two different angles: synthetic biology changes bodies, and architecture changes environments. At the recent conference “Genetics, Biomedicine, and the Human Experience in Space,” you presented ideas about using genetics to do things that are normally done by altering environments—for instance, by changing our microbi- ome, we might not have to worry as much about creating sterile environments for surgery. Can geneticists change our environments in ways that architects can’t? And vice versa?
George Church
Most of the things that we’ve already changed in our environments don’t strike me as great candidates for genetics. Our ability to go to Mars by packing ourselves into metal containers and shooting them through space is environmental manipulation—it would be difficult to do that using genetics. Of course there are smaller changes we could make genetically: preparing ourselves for zero gravity, getting better at radiation resistance, and maybe even handling all of our pathogens. But the basic problems—transportation, our ability to survive in extreme temperatures—are easy to do with physics but hard to do with genetics.
What could synthetic biology do better than architecture? I think architecture is fairly weak at impacting disease. We could all live in little glass bubbles and stop interacting with other people, and that would solve some infectious diseases, but it would probably be easier to do genetically: we could make ourselves resistant to all viruses and eliminate all environmental sources of plagues.
MA
You’ve done experiments to eliminate malaria using gene drives to change entire mosquito populations …
GC
… which is much more effective than using netting. Anything that’s native to our biology is probably best done biologically.
How computing is best done is an open question. Is it best done with an architecture of silicon or by improving our brains? Right now, development in biological technology is on a faster curve than Moore’s law. That pathetic machine, Watson, developed by IBM to compete on the quiz show Jeopardy!, used 200,000 watts, as opposed to the 20 watts that the brain uses. The brain is more energy efficient by far—and about a billion times more compact.
MA
The revolution in synthetic biology has been characterized as the shift from reading DNA to writing DNA. You pioneered earlier phases of genomics—in 1994 you worked on the first genome sequencer ever—and today you are at the forefront of figuring out how to design and build genomes. How do you go about designing a genome? What is the process?
GC
The synthetic biology revolution is not just about going from reading to writing. Genomics already went from reading single genes to reading multiple genes; now synthetic biology is going from writing single genes to writing whole genomes. Both reading and writing are tangled up in the design process. In many fields, there is a design-build-analyze loop: you build something, and then you look for its failure modes. After living in a building for a while, you notice that it leaks. Then you do another round of design; you radicalize your structures and hold them to stronger standards until they fail. Then you slowly eek your way back to something that works, but works better than before. The same thing is true in synthetic biology. We have design software, like BIOCAD, cadnano, Millstone, and others.
But I see two fundamental differences between synthetic biology and architecture. In architecture, you might start with walls and windows as your standard parts. In biology, our standard parts have been refined by three billion years of evolution, on 1021 liters of soil and water. That’s a lot of debugging. Also, in synthetic biology we have the ability to recreate that refinement process ourselves, on a smaller scale and in a more directed way. We can run our own evolutions. When you do the design-build-analyze loop for buildings, you might make one small prototype, build it, and, if it starts to go wrong, you debug it in real time. Like the John Hancock Tower, in downtown Boston—you know its history, right?
MA
Glass panels mysteriously falling off …
GC
It was being debugged as it was being used. With synthetic biology, we can make a billion or a trillion designs, build them all, test them all, take the winner from that testing, and then do it all again.
MA
What is the timescale for this type of experiment?
GC
It depends on your goal. If your goal is to make a chemical, say, or to build a little factory that makes chemicals, you can design, build, and test a billion things in one day. If your goal is to make a pig, you’re talking more in the order of years. And if you are creating a human pharmaceutical, you’re talking about 10 years just to get it through all the regulatory phases. You might find a clever way of doing billions of prototypes by working with human cells in the lab, but when you want to introduce it into the marketplace, you’re going to be testing one drug at a time, just like you test one building at a time.
MA
You’ve worked on some things that are pretty far removed from our daily concerns—like how to bring the wooly mammoth back to life—but a lot of your work stands to affect our everyday bodily experience. What are you working on that you might want to use to change your own genome?
GC
There is an APP (amyloid precursor protein) allele that I wouldn’t mind having—it gives an extra 10 years of resistance to Alzheimer’s. That’s something that’s preventative, and it’s something we more or less know how to do. But there are some things we don’t know how to do yet, such as having better memory or making more effective use of the brain. Those would be great. Reversing aging would be nice, too.
MA
Aren’t our inadequacies part of what makes us human? How would it affect the human experience if we could live much longer, for example?
GC
I think what makes us human is mainly our ability to plan and to care for others. Chimpanzees form little cliques, and they certainly care for their families, but I think our ability to imagine scenarios that have never happened—to think of ways to avoid having an asteroid eliminate all life on the planet—is uniquely human. We have an ability to be thoughtful about ourselves and oth- ers over long periods of time. I think that would remain true if we lived longer.
MA
Your lab has been helping make genetic sequencers much smaller and less expensive, potentially democratizing and distributing biological research. How is design at the level of personal products, like wearable devices, changing the discipline of biology and the health-care system?
GC
Our lab is dedicated to democratization—we are radically open. We do open-source human biology: we give away our cells, we give away all our medical records, and so on. We also radically bring down costs. It’s one thing to make a plastic that’s, say, 20 percent cheaper than market rate. That’s important, but sometimes you need to bring down costs a million-fold to turn a big-science toy into something that people in the developing world can use. We’ve helped bring down the cost of genetic sequencing dramatically; the cost of one genome has gone from $3,000,000,000 to about $800. And I hope we can drive it down to the dollar range. That’s what happened in telecommunications: it used to be extraordinarily expensive for rich people to phone across the Atlantic; now, people in the developing world are using their cellphones for microfinancing.
You have to ask, how can we change everything we’re doing? My lab has designed a whole series of instruments for reading and writing DNA. We’re developing sequencers that are small enough, and have low enough energy consumption, that they can be worn on the body and can monitor the environment constantly. So it might tell you if you’re entering a zone where there’s something that you’re allergic to, or that is a threat to everybody. Your device is going to filter information and tell you only what you need to know. Just like GPS navigation: it goes through mountains of GPS data and says, Turn left. Your sequencer might filter mountains of bioweather data and say, Don’t go right, because there’s H1N1 to the right. That’s not just democratization and decentralization; that’s dropping the bottom out of entire markets.
MA
Another open-source biology project you are running, the Personal Genome Project, is the first project of its kind not only to put together a database of genomes, but to pair them with information about traits and environments. What is the environmental data you collect?
GC
One data set is microbiomes. Your microbiome can tell who you are—all the genetic sequences of the bacteria and other microorganisms that live inside you—and also what you’ve been exposed to.
MA
Are you moving toward some type of “life recording”— using multiple sensors to record everything happening in people’s environments, at all times?
GC
Yes, this is already happening. One thing we’re devel- oping in the Personal Genome Project is a combined multiple sensor to put into eyeglasses. From one point on someone’s head, we can get a lot of information—not only what they’re seeing; but we can also get an EKG, EEG, and so on. Having 24/7 monitoring of both environment and internals is very valuable because a lot of things happen outside of the physician’s office and it’s not clear what sets them off. If you do an EKG or EEG in a physician’s office, you may see nothing, but if you’re wearing a monitor and have an epilepsy attack, or a narcolepsy attack, or a cardiac arrhythmia … Was it what you were eating that set these things off? Was it what you were eating that set things off? Was there a flashing light that set it off? All this stuff could be integrated. And if you can make that technology accessible, you are going to start solving medical problems left and right.
MA
Synthetic biology has extraordinarily grand ambitions—not only to change molecules, but to change humanity itself. You could even characterize the synthetic biologist as the ultimate designer. If all of your plans were to work out, what would the consequences be? What is your vision for the future?
GC
You describe synthetic biology as the ultimate design. But what does “ultimate” mean? To me, ultimate would mean being able to design with atomic precision, but at grand scales, and to some extent in any medium. That’s not where we are with synthetic biology, but part of the vision is that we could get there.
Certainly biology is both grand and almost atomically precise. There are trees—giant redwoods—that are grand, but if you look at any piece of them, you see endless variations on the theme of how to put xylem and phloem in the right places to be able to take water up beyond the capillary limits. Plant life also sculpted the atmosphere: the atmosphere is full of oxygen, a product of photosynthesis. When you look at the earth from outer space, you see the impact of oxygen on the blueness of the air. That’s biology—not synthetic biology.
So what could we do with synthetic biology? Synthetic biologists should be able to make computing machines that are better than the current pinnacle of computing machines—the human brain. We should be able to cure enough disease and poverty and war-like behavior to have some excess wealth. With that, we can head into outer space, to every potentially habitable planet. Otherwise we are dead. Our only chance at survival as a species is to conquer our limitations, which are a result of living in poverty. The entire planet is entangled in wars between rich and poor.
MA
So in the long run, you’re counting on the human brain?
GC
Yes. The human brain is an amazing piece of architecture, even more amazing than the redwood tree. People glibly talk about artificial intelligence; we put a type of human intelligence that is actually machine intelligence on a pedestal. But before we had machines it was considered intelligent if you could recite the Iliad and the Odyssey without looking at notes. That was our premonition of what machines would be able to do. But the really amazing thing about humans is to have written the Iliad and the Odyssey in the first place, or to have conceived of getting in a little boat and going across the Pacific Ocean without knowing what was there.
MA
What are the next steps? You’ve been involved with projects that are mapping all the connections in someone’s brain—the connectome. Is the goal to build one artificially?
GC
What we want is a design-build-test loop. If we just observe the connectome and the transcriptome and all the -omes of the brain, we can build elaborate models—castles in the sky that won’t mean anything until they’re tested. Conversely, you can do tests and build things without fully understanding how they work. We need to take a leap and try all these things. This is how we figured out how vaccines work. We went through a century doing vaccines without knowing anything about immunology; we knew squat about B-cells, T-cells, and cytokines when we did the first vaccines, and the hundredth vaccines. But we knew a basic concept and we were willing to take that leap—like taking a little dingy out on the Pacific Ocean. I think the same thing will happen with the brain—as soon as we get accustomed to reading and building neurocircuits.
Our most advanced design-build-analyze tests have been to take stem cells—actually some cells from my left arm—and turn them into neurons. We can make them take many different neuronal pathways, arrange them, and monitor them. We’re trying, in particular, to make hippocampal circuits; the hippocampus is where our memories are, and we want to see if we can build bigger memories. We don’t just want to build bigger memories, we want emotional intelligence, out-of-the-box thinking, and so on. But I think hippocampal circuits are a place to start.
MA
What has happened when you’ve been able to grow them? Can you just grow these things and plug them into somebody’s brain?
GC
Well, some people are in need—people with epilepsy might have a cubic centimeter removed, and people with Parkinson’s will have things implanted. But the brain is probably one of the least ideal tissues for transplants because the cells are far from spherical and the organ is far from modular. With a kidney it’s pretty modular, but in the brain, each cell has arms that are thousands of times the length of the nucleus. But it can be done, especially at an embryonic stage.
It’s going to happen in baby steps, and it’s going to start with people that are particularly needy. But it will quickly transition as soon as there is evidence that it is effective. Medicine is a very reactive field; it’s about safety and advocacy. As soon as you solve those problems, the door is wide open.
People get confused about ethics. Ethics is typically about the way things are, not the way things could be. As soon as things change, then ethics change—sometimes 180 degrees. This happened with in vitro fertilization. There were all these scare tactics about test-tube babies; even the term “test-tube baby” was pejorative, conjuring up genetic monsters. But then the first one was born and suddenly the ethics changed.
MA
This goes back to the idea that what we take as natural is highly artificial, and that it doesn’t actually take long for something to become natural.
GC
That’s right. Like my natural car, or my natural home. Even things that are considered anachronistic or prehistoric, like an axe or a wood-burning stove, are extraordinarily unnatural!
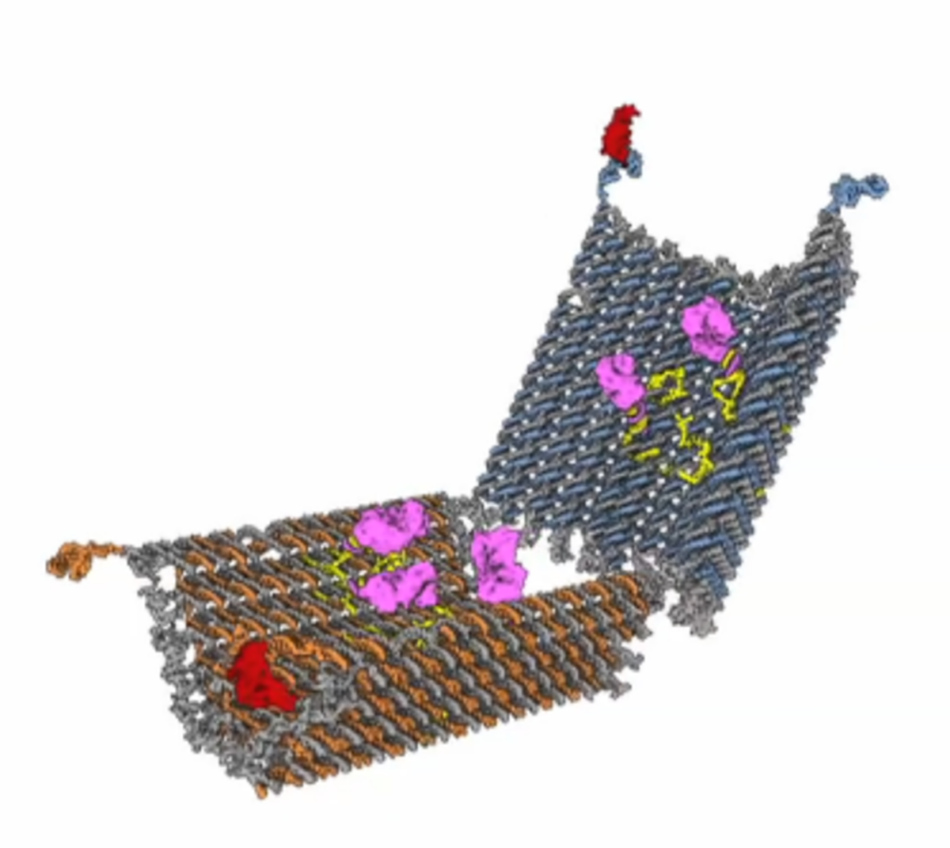
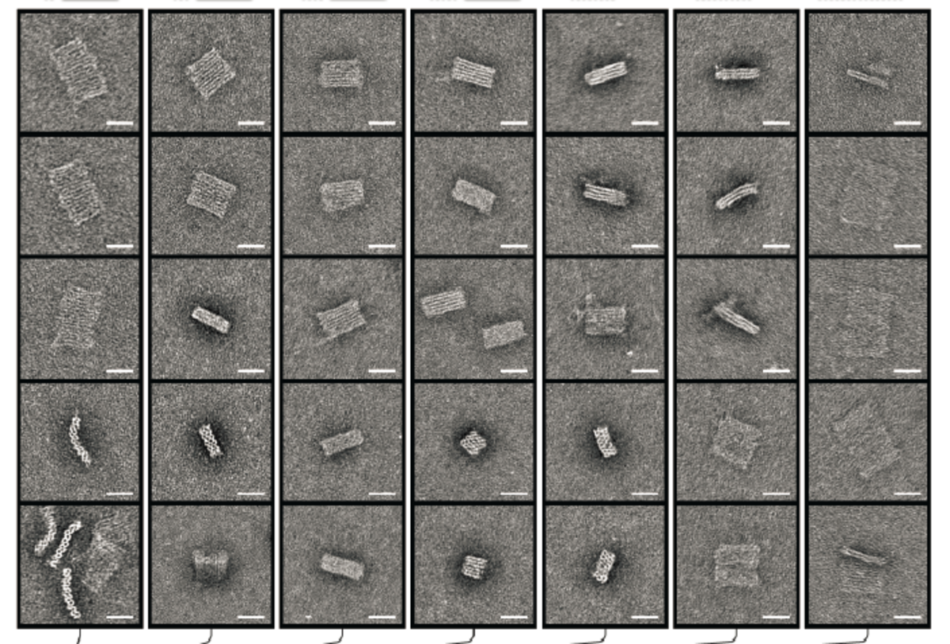
George Church is Professors of Genetics at Harvard Medical School, director of the Personal Genome Project, and cofounder of several commercial enterprises invested in human genomics, green chemistry, and solar fuel. A pioneer in the field of genome sequencing and open-source genomic data, he is a member of the National Academy of Sciences and the National Academy of Engineering, and is coauthor of many papers, patents, and the book Regenesis: How Synthetic Biology Will Reinvent Nature and Ourselves (2014) with Ed Regis.
Matthew Allen is a PhD candidate at the Harvard University Graduate School of Design who studies cognitive prosthetics—how architects think and the devices that help them. He has taught at the University of Toronto and has published articles in Domus and Log, among other journals.